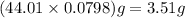Gaseous methane (CH4) reacts with gaseous oxygen (O2) gas to produce gaseous carbon dioxide (CO2) and gaseous water (H2O). What is the theoretical yield of carbon dioxide formed from the reaction of 1.28 g of methane and 10.1 g of oxygen gas? Be sure your answer has the correct number of significant digits in it.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1


Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
You know the right answer?
Gaseous methane (CH4) reacts with gaseous oxygen (O2) gas to produce gaseous carbon dioxide (CO2) an...
Questions




Mathematics, 07.11.2020 22:50



English, 07.11.2020 22:50

Law, 07.11.2020 22:50

Chemistry, 07.11.2020 22:50


Mathematics, 07.11.2020 23:00



Mathematics, 07.11.2020 23:00

Mathematics, 07.11.2020 23:00



Health, 07.11.2020 23:00


Computers and Technology, 07.11.2020 23:00

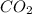 is 3.51 g.
is 3.51 g.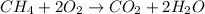
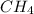 = 16.04 g/mol
= 16.04 g/mol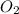 = 32.00 g/mol
= 32.00 g/mol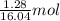 of
of  of
of