
Chemistry, 07.04.2020 21:50 hunteryolanda82
The Kb of weak base B is 1.0 x 10-6. A solution contains 0.75 M B and 0.25 M HB+Cl- (the salt of B with HCl). What is the pH of the solution after 0.05 mol NaOH is added to 1.0 L of the above solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
The Kb of weak base B is 1.0 x 10-6. A solution contains 0.75 M B and 0.25 M HB+Cl- (the salt of B w...
Questions



Mathematics, 06.03.2021 23:00

Physics, 06.03.2021 23:00

Mathematics, 06.03.2021 23:00


Biology, 06.03.2021 23:00


Social Studies, 06.03.2021 23:00

Mathematics, 06.03.2021 23:00

Mathematics, 06.03.2021 23:00

Business, 06.03.2021 23:00

History, 06.03.2021 23:00



Mathematics, 06.03.2021 23:00

Health, 06.03.2021 23:00



Mathematics, 06.03.2021 23:00

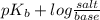
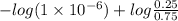
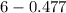
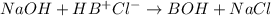
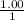 , [Salt] =
, [Salt] = 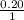
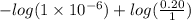 [/tex]
[/tex]

