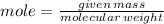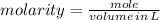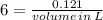To produce 40.0 g of silver chromate, you will need at least 23.4 g of potassium chromate in solution as a reactant. All you have on hand in the stock room is 5 L of a 6.00 M K2CrO4 solution. What volume of the solution is needed to give you the 23.4 g K2CrO4 needed for the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

You know the right answer?
To produce 40.0 g of silver chromate, you will need at least 23.4 g of potassium chromate in solutio...
Questions




Mathematics, 19.02.2021 05:00

Mathematics, 19.02.2021 05:00

Mathematics, 19.02.2021 05:00

Mathematics, 19.02.2021 05:00



Social Studies, 19.02.2021 05:00

Mathematics, 19.02.2021 05:00

Mathematics, 19.02.2021 05:00

Biology, 19.02.2021 05:00

Spanish, 19.02.2021 05:00






Mathematics, 19.02.2021 05:00

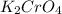 required=20.1 ml
required=20.1 ml