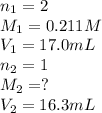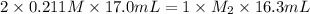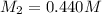Chemistry, 07.04.2020 21:20 jadbaubl1449
A titration reached the equivalence point when 17.0 mL of was added to of NaOH (aq) of unknown concentration. What is the concentration (M) of this unknown NaOH solution? H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 03:00
Air pressure is measured in pascals. for a professional american football game, the ball should be inflated to about 90,000 pascals. scientists studied the effects of air temperature on the pressure inside american footballs by taking these steps: 1. prepare 100 footballs. 2. measure each football's air pressure. 3. divide footballs into 10 groups. 4. place the groups in different lockers cooled to different air temperatures. 5. after 12 hours, remove the footballs from lockers. 6. measure each football's pressure again. 7. compare the new pressures to the starting pressures. what two terms best describe the variable "air pressure inside the football" in this experiment? independent, qualitative independent, quantitative dependent, qualitative dependent, quantitative
Answers: 3
You know the right answer?
A titration reached the equivalence point when 17.0 mL of was added to of NaOH (aq) of unknown conce...
Questions

Mathematics, 17.10.2020 14:01


Mathematics, 17.10.2020 14:01



Mathematics, 17.10.2020 14:01

Health, 17.10.2020 14:01



Mathematics, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01








Mathematics, 17.10.2020 14:01

Biology, 17.10.2020 14:01

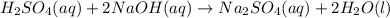
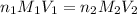
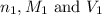 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 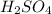
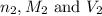 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.