

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
When the experiment was carried out, the temperature was 22 oC and atmospheric pressure was 1.007 at...
Questions

Mathematics, 02.07.2021 01:00






Mathematics, 02.07.2021 01:00

Mathematics, 02.07.2021 01:00


Mathematics, 02.07.2021 01:00

Mathematics, 02.07.2021 01:00


Mathematics, 02.07.2021 01:00

Health, 02.07.2021 01:00




Computers and Technology, 02.07.2021 01:00


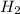 =4.16mol
=4.16mol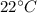 =273+22 k=295k
=273+22 k=295k

