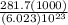Chemistry, 07.04.2020 22:28 mmassaro19
It takes 281.7 kJ of energy to remove 1 mole of electrons from the atoms on the surface of lithium metal. If lithium metal is irradiated with 261-nm light, what is the maximum kinetic energy the released electrons can have

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
This line graph compares the growth of plants that were kept in the sun for different amounts of time.a) on day 7, the plants kept in the sun for 3 hours were how tall? b) on day 7, the plants kept in the sun for 6 hours were how tall? c) on day 10, the plants kept in the sun for 9 hours were how tall? d) on day 11, the plant that was grown with 1 hour of sunlight was how tall? e) based on the graph, the plant grows best in what amount of sunlight?
Answers: 1


Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1
You know the right answer?
It takes 281.7 kJ of energy to remove 1 mole of electrons from the atoms on the surface of lithium m...
Questions



Mathematics, 18.08.2021 20:20




History, 18.08.2021 20:20

Mathematics, 18.08.2021 20:20






Geography, 18.08.2021 20:20




History, 18.08.2021 20:30


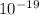 Joule
Joule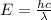 ------------ (1)
------------ (1)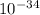 J s
J s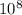
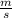
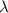 = 261 nm = 261 ×
= 261 nm = 261 × 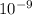 m
m