
Chemistry, 07.04.2020 23:36 rebeccamckellpidge
The combustion of propane gas is used to fuel barbeque grills. If 4.65 moles of propane, C3H8, are burned in a grilling session, how many moles of carbon dioxide gas are formed? C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(l)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3


Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1
You know the right answer?
The combustion of propane gas is used to fuel barbeque grills. If 4.65 moles of propane, C3H8, are b...
Questions





English, 20.10.2020 22:01



Chemistry, 20.10.2020 22:01

Physics, 20.10.2020 22:01


Spanish, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01


Mathematics, 20.10.2020 22:01

Chemistry, 20.10.2020 22:01


English, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01


Physics, 20.10.2020 22:01

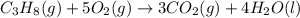
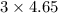 moles of carbon dioxide will form
moles of carbon dioxide will form 

