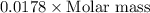Chemistry, 07.04.2020 22:58 alyssahomeworkneeds
Given the following information: 1.6 g of an unknown monoprotic acid (HA) required 50.80 mL of a 0.35 M NaOH solution to reach the equivalence point, calculate the molar mass (g/mol) of the acid. Enter the value ONLY. Do not include the units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
Given the following information: 1.6 g of an unknown monoprotic acid (HA) required 50.80 mL of a 0.3...
Questions

Mathematics, 25.01.2021 18:10

Mathematics, 25.01.2021 18:10


History, 25.01.2021 18:10


Mathematics, 25.01.2021 18:10





Physics, 25.01.2021 18:10








Biology, 25.01.2021 18:10

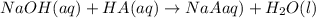
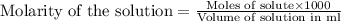 .....(1)
.....(1)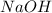 solution = 0.35 M
solution = 0.35 M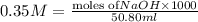

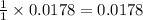 moles of HCl
moles of HCl