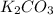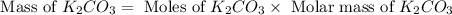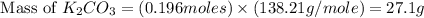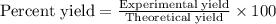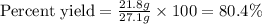Chemistry, 07.04.2020 22:58 timjape3g3z
Determine the theoretical yield and the percent yield if 21.8 g of KCO is produced from reacting 27.9 g KO with 29.0 L of CO (at STP). The molar mass of KO = 71.10 g/mol and KCO = 138.21 g/mol. 4 KO(s) + 2 CO(g) → 2 KCO(s) + 3 O(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1


Chemistry, 23.06.2019 13:30
Which factors would have influenced earth’s climate during the time of pangea? check all that apply. land covered by glaciers one large landmass one large ocean tectonic activity multiple small seas
Answers: 2
You know the right answer?
Determine the theoretical yield and the percent yield if 21.8 g of KCO is produced from reacting 27....
Questions

English, 23.11.2020 18:50



Mathematics, 23.11.2020 18:50

Physics, 23.11.2020 18:50

Biology, 23.11.2020 18:50

Mathematics, 23.11.2020 18:50




History, 23.11.2020 18:50

Mathematics, 23.11.2020 18:50


Mathematics, 23.11.2020 18:50

Mathematics, 23.11.2020 18:50

Mathematics, 23.11.2020 18:50


English, 23.11.2020 18:50

Mathematics, 23.11.2020 18:50

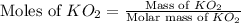
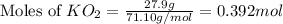
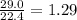 mole of CO₂ gas.
mole of CO₂ gas.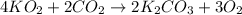
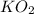 react with 2 mole of
react with 2 mole of 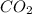
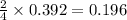 moles of
moles of