
The equilibrium expression for a reaction is keq=[h+]6[bi2+]2[h2s]3. which of the following could be the reaction? a. 6h+(aq) + bis(s) 2bi2+(aq) + 3h2s(g) b. 2bi2+(aq) + 3h2s(aq) bi2s3(s) + 6h+(aq) c. 6h+(aq) + bi2s3(s) 2bi2+(aq) + 3h2s(g) d. 2bi2+(aq) + 3h2s(aq) bi2s3(aq) + 6h+(aq)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
The equilibrium expression for a reaction is keq=[h+]6[bi2+]2[h2s]3. which of the following could be...
Questions


Mathematics, 13.11.2020 01:00






Arts, 13.11.2020 01:00

Physics, 13.11.2020 01:00

Arts, 13.11.2020 01:00

Arts, 13.11.2020 01:00




History, 13.11.2020 01:00




Mathematics, 13.11.2020 01:00

Biology, 13.11.2020 01:00

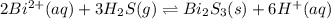
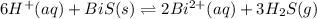
![k_{eq}=\frac{[Bi^{2+}]^2[H_2S]^3}{[H^+]^6}](/tpl/images/0354/4031/90ef6.png)
![k_{eq}=\frac{[H^{+}]^6}{[Bi^{2+}]^2[H_2S]^3}](/tpl/images/0354/4031/d24c1.png)
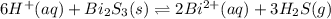
![k_{eq}=\frac{[Bi^{2+}]^2[H_2S]^3}{[H^{+}]^6}](/tpl/images/0354/4031/14e9d.png)
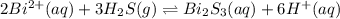
![k_{eq}=\frac{[H^{+}]^6[Bi_2S_3]}{[Bi^{2+}]^2[H_2S]^3}](/tpl/images/0354/4031/e2680.png)


