Chemistry, 07.04.2020 23:18 tynyiaawrightt
An automobile engine provides 639 Joules of work to push the pistons and generates 2194 Joules of heat that must be carried away by the cooling system. Calculate the change in the internal energy of the engine. E = Joules

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

You know the right answer?
An automobile engine provides 639 Joules of work to push the pistons and generates 2194 Joules of he...
Questions

Mathematics, 11.10.2021 09:00



Mathematics, 11.10.2021 09:00






Mathematics, 11.10.2021 09:00



Mathematics, 11.10.2021 09:00


Mathematics, 11.10.2021 09:00





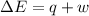
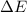 = Change in internal energy
= Change in internal energy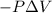 = -639 J {Work is done by the system and is negative as the final volume is greater than initial volume }
= -639 J {Work is done by the system and is negative as the final volume is greater than initial volume }