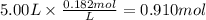The reaction 2N2O5 (g) → 2N2O4 (g) + O2 (g) has a reaction rate that is dependent only on the concentration of N2O5 and at a certain temperature has a rate constant k of 0.0168 s-1. If 2.50 moles of N2O5 were placed in a 5.00 liter container at that temperature, how many moles of N2O5 would remain after 1.00 min?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

You know the right answer?
The reaction 2N2O5 (g) → 2N2O4 (g) + O2 (g) has a reaction rate that is dependent only on the concen...
Questions

Engineering, 06.04.2021 18:10

Mathematics, 06.04.2021 18:10

Mathematics, 06.04.2021 18:10




Mathematics, 06.04.2021 18:10


English, 06.04.2021 18:10




Biology, 06.04.2021 18:10






Mathematics, 06.04.2021 18:10

Mathematics, 06.04.2021 18:10

![[N_2O_5] = [N_2O_5]_0 \times e^{-k \times t}](/tpl/images/0587/8861/ec7f1.png)
![[N_2O_5]_0](/tpl/images/0587/8861/4a20d.png) : initial concentrationk: rate constantt: time
: initial concentrationk: rate constantt: time![[N_2O_5] = 0.500 M \times e^{-0.0168 s^{-1} \times 60s} = 0.182 M](/tpl/images/0587/8861/ce720.png)