
Chemistry, 07.04.2020 23:53 audrey1256
1. Sulfuric acid is formed when sulfur dioxide gas reacts with oxygen gas and water. Write a balanced chemical equation for the reaction. If 12.5 mol sulfur dioxide reacts, how many mol of sulfuric acid can be produced and how many moles of oxygen gas is needed

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 23.06.2019 11:00
What are the other two pieces of glassware you used in this experiment that you could obtain hundredths digit accuracy?
Answers: 2

Chemistry, 23.06.2019 11:30
How many grams of carbon are in 237 grams of ethanol(c2h5oh) and how many sulfide ions are in 2.45 moles of aluminum sulfide show me you you got the answers
Answers: 3
You know the right answer?
1. Sulfuric acid is formed when sulfur dioxide gas reacts with oxygen gas and water. Write a balance...
Questions

Biology, 30.03.2021 03:20

Mathematics, 30.03.2021 03:20




Mathematics, 30.03.2021 03:20



Mathematics, 30.03.2021 03:20

Mathematics, 30.03.2021 03:20


Mathematics, 30.03.2021 03:20


Mathematics, 30.03.2021 03:20

Spanish, 30.03.2021 03:20



Mathematics, 30.03.2021 03:20

Mathematics, 30.03.2021 03:20

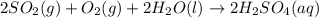
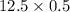 mole of oxygen
mole of oxygen

