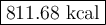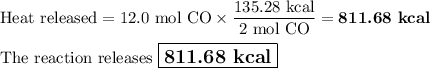Chemistry, 07.04.2020 23:54 hardwick744
Carbon monoxide reacts with oxygen to form carbon dioxide by the following reaction: 2 CO(g) + O2(g) → 2 CO2(g) ∆H for this reaction is −135.28 kcal. How much heat would be released if 12.0 moles of carbon monoxide reacted with sufficient oxygen to produce carbon dioxide?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2


Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

You know the right answer?
Carbon monoxide reacts with oxygen to form carbon dioxide by the following reaction: 2 CO(g) + O2(g)...
Questions


Mathematics, 20.09.2020 18:01

Mathematics, 20.09.2020 18:01



Biology, 20.09.2020 18:01










Mathematics, 20.09.2020 18:01




Mathematics, 20.09.2020 18:01