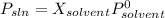Chemistry, 07.04.2020 23:47 mariamsakayanthebest
At a certain temperature, the vapor pressure of pure benzene is atm. A solution was prepared by dissolving g of a nondissociating, nonvolatile solute in g of benzene at that temperature. The vapor pressure of the solution was found to be atm. Assuming that the solution behaves ideally, determine the molar mass of the solute.

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

Chemistry, 23.06.2019 06:30
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2
You know the right answer?
At a certain temperature, the vapor pressure of pure benzene is atm. A solution was prepared by di...
Questions

Mathematics, 22.05.2020 19:08

Mathematics, 22.05.2020 19:08


Mathematics, 22.05.2020 19:08


Mathematics, 22.05.2020 19:08

Mathematics, 22.05.2020 19:08

Mathematics, 22.05.2020 19:08


Geography, 22.05.2020 19:57


Mathematics, 22.05.2020 19:57

History, 22.05.2020 19:57



Mathematics, 22.05.2020 19:57


Mathematics, 22.05.2020 19:57

History, 22.05.2020 19:57

Chemistry, 22.05.2020 19:57