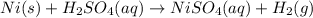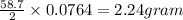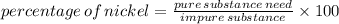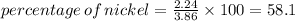Chemistry, 08.04.2020 01:38 dancemomsrule1
III. (4 points) A sample of nickel ore, which has nickel as the only metal present, is treated with an excess of sulfuric acid (H2SO4) to form nickel(II) sulfate and molecular hydrogen. (a) Write a balanced equation for the reaction. (b) If 0.0764 g of H2 is obtained from 3.86 g of the nickel ore, calculate the percent nickel, by mass, of the nickel ore.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1


Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2
You know the right answer?
III. (4 points) A sample of nickel ore, which has nickel as the only metal present, is treated with...
Questions

History, 10.10.2019 20:30

Mathematics, 10.10.2019 20:30

Mathematics, 10.10.2019 20:30

Mathematics, 10.10.2019 20:30



Mathematics, 10.10.2019 20:30

History, 10.10.2019 20:30

History, 10.10.2019 20:30

Computers and Technology, 10.10.2019 20:30

Physics, 10.10.2019 20:30




English, 10.10.2019 20:30


English, 10.10.2019 20:30

Mathematics, 10.10.2019 20:30