
9. Diiodine pentoxide is useful in devices such as respirators because it reacts with the dangerous gas carbon monoxide, CO, to produce relatively harmless CO2 according to the following equation: I2O5 5CO £ I2 5CO2 a. In testing a respirator, 2.00 g of carbon monoxide gas is passed through diiodine pentoxide. Upon analyzing the results, it is found that 3.17 g of I2 was produced. Calculate the percentage yield of the reaction. b. Assuming that the yield in (a) resulted because some of the CO did not react, calculate the mass of CO that passed through.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1
You know the right answer?
9. Diiodine pentoxide is useful in devices such as respirators because it reacts with the dangerous...
Questions

History, 08.07.2019 23:00

Mathematics, 08.07.2019 23:00

History, 08.07.2019 23:00








English, 08.07.2019 23:00

English, 08.07.2019 23:00


History, 08.07.2019 23:00


English, 08.07.2019 23:00

Physics, 08.07.2019 23:00


French, 08.07.2019 23:00

Geography, 08.07.2019 23:00

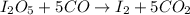
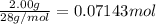
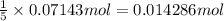 of iodine gas
of iodine gas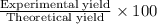
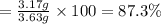
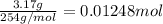
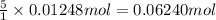 of carbon monoxide
of carbon monoxide

