
While performing the Acid-catalyzed Hydrolysis of Epoxides experiment you used a solution containing 1.84 mL of cyclohexene oxide. After the reaction was completed and worked up, you obtained 0.739 g of 1,2-cyclohexane diol. Calculate the moles of starting material used.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
While performing the Acid-catalyzed Hydrolysis of Epoxides experiment you used a solution containing...
Questions

Chemistry, 02.12.2019 08:31



Mathematics, 02.12.2019 08:31

History, 02.12.2019 08:31

Mathematics, 02.12.2019 08:31

Mathematics, 02.12.2019 08:31


Mathematics, 02.12.2019 08:31




Mathematics, 02.12.2019 08:31

Mathematics, 02.12.2019 08:31



Biology, 02.12.2019 08:31

Physics, 02.12.2019 08:31


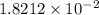 mole
mole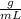
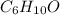
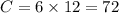
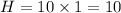
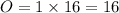
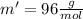
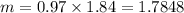
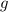
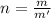
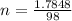
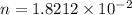 mol
mol

