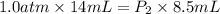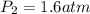Asnorkeler takes a syringe filled with 14ml of air from the surface, where the pressure is 1.0 atm, to an unknown depth. the volume of the air in the syringe at this depth is 8.5ml. a) what is the pressure at this depth? b) if the pressure increases by 1 atm for every additional 10 m of depth, how deep is the snorkeler?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1


You know the right answer?
Asnorkeler takes a syringe filled with 14ml of air from the surface, where the pressure is 1.0 atm,...
Questions

History, 07.07.2019 10:10

History, 07.07.2019 10:10



Chemistry, 07.07.2019 10:10

Biology, 07.07.2019 10:10

Mathematics, 07.07.2019 10:10


Biology, 07.07.2019 10:10





English, 07.07.2019 10:10


History, 07.07.2019 10:10

Mathematics, 07.07.2019 10:10



English, 07.07.2019 10:10

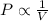
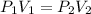
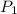 = initial pressure = 1.0 atm
= initial pressure = 1.0 atm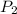 = final pressure = ?
= final pressure = ?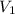 = initial volume = 14 mL
= initial volume = 14 mL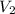 = final volume = 8.5 mL
= final volume = 8.5 mL