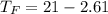Chemistry, 08.04.2020 01:15 brianna8739
Commercial cold packs consist of solid ammonium nitrate and water. NH 4NO 3( s) absorbs 25.69 kJ of heat per mole dissolved in water. In a coffee-cup calorimeter, 3.40 g NH 4NO 3( s) is dissolved in 100.0 g of water at 21.0 °C. What is the final temperature of the solution? Assume that the solution has a specific heat capacity of 4.18 J/g•K.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Commercial cold packs consist of solid ammonium nitrate and water. NH 4NO 3( s) absorbs 25.69 kJ of...
Questions



Mathematics, 09.07.2021 16:50

Mathematics, 09.07.2021 16:50

Mathematics, 09.07.2021 16:50








English, 09.07.2021 16:50

Mathematics, 09.07.2021 16:50

Geography, 09.07.2021 16:50



English, 09.07.2021 16:50


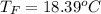
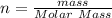
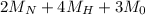
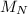 (molar mass of Nitrogen) , 1 for
(molar mass of Nitrogen) , 1 for 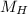 molar mass of hydrogen, 16 for
molar mass of hydrogen, 16 for 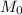 molar mass of oxygen
molar mass of oxygen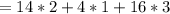
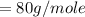
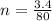
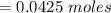

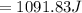
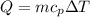
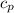 (Specific heat capacity)
(Specific heat capacity) the subject
the subject 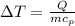
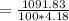
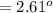 C
C