
Chemistry, 08.04.2020 01:33 imeldachavez124
He thermite reaction, in which powdered aluminum reacts with iron(III) oxide, is highly exothermic. 2 Al(s) + Fe2O3(s) Al2O3(s) + 2 Fe(s) Use standard enthalpies of formation to find for the thermite reaction

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3

Chemistry, 23.06.2019 13:00
Which of the following statements is true about both nuclear fusion and nuclear fission? they occur in the sun. heavy atoms are split. two light nuclei combine. some mass changes into energy.
Answers: 1

Chemistry, 23.06.2019 16:50
Equal volumes of h2 and o2 are placed in a balloon and then ignited. assuming that the reaction goes to completion, which gas will remain in excess? 2h2 + o2 → 2h2o a. both are in equal proportion b. oxygen c. water d. hydrogen
Answers: 1
You know the right answer?
He thermite reaction, in which powdered aluminum reacts with iron(III) oxide, is highly exothermic....
Questions

Mathematics, 02.02.2021 21:30


Law, 02.02.2021 21:30


Mathematics, 02.02.2021 21:30

Mathematics, 02.02.2021 21:30





Mathematics, 02.02.2021 21:30


History, 02.02.2021 21:30

History, 02.02.2021 21:30



Mathematics, 02.02.2021 21:30

History, 02.02.2021 21:30

Mathematics, 02.02.2021 21:30

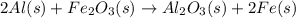 . Use standard enthalpies of formation to find ΔH∘rxn for the thermite reaction. Express the heat of the reaction in kilojoules to four significant figures.
. Use standard enthalpies of formation to find ΔH∘rxn for the thermite reaction. Express the heat of the reaction in kilojoules to four significant figures.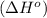 .
.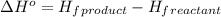
![\Delta H^o=[n_{Fe}\times \Delta H_f^0_{(Fe)}+n_{Al_2O_3}\times \Delta H_f^0_{(Al_2O_3)}]-[n_{Al}\times \Delta H_f^0_(Al)+n_{Fe_2O_3}\times \Delta H_f^0_{(Fe_2O_3)}]](/tpl/images/0588/4390/ac2a9.png)
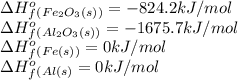
![\Delta H^o_{rxn}=[(2\times 0)+(1\times -1675.5)]-[(2\times 0)+(1\times -824.2)]=-851.5kJ](/tpl/images/0588/4390/65523.png)


