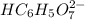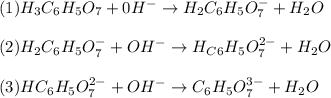Chemistry, 08.04.2020 01:44 jayline2003
: Citric acid, H3C6H5O7, is a triprotic acid. Consider a buffer system comprising H2C6H5O7 - and HC6H5O7 2- ions. What is the net ionic equation for the reaction that occurs when NaOH is added to a buffer containing H2C6H5O7 - and HC6H5O7 2- ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
: Citric acid, H3C6H5O7, is a triprotic acid. Consider a buffer system comprising H2C6H5O7 - and HC6...
Questions





Social Studies, 10.11.2020 22:00


Mathematics, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00

Physics, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00

History, 10.11.2020 22:00

Mathematics, 10.11.2020 22:00


English, 10.11.2020 22:00


Mathematics, 10.11.2020 22:00


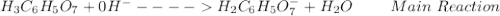
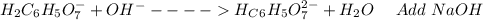
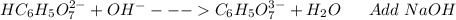
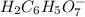 and
and