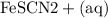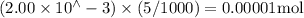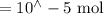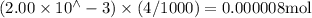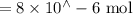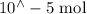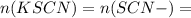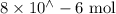Suppose a student mixes 4.00 mL of 2.00 # 10-3 M Fe(NO3 ) 3 with 5.00 mL of 2.00 # 10-3 M KSCN and 1.00 mL of water. The student then determines the [FeNCS2+] at equilibrium to be 8.75 # 10-5 M. Find the equilibrium constant for the following reaction. Show all your calculations for each step. Fe3+ (aq) + SCN- (aq) FeNCS2+ (aq) Step 1. Calculate the initial number of moles of Fe3+ and SCN- (use Equation 12). moles of Fe3+ moles of SCN- Step 2. How many moles of FeNCS2+ are present at equilibrium? What is the volume of the equilibrium mixture? mL moles of FeNCS2+ How many moles of Fe3+ and SCN- are consumed to produce the FeNCS2+? moles of Fe3+ moles of SCN-

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3


You know the right answer?
Suppose a student mixes 4.00 mL of 2.00 # 10-3 M Fe(NO3 ) 3 with 5.00 mL of 2.00 # 10-3 M KSCN and 1...
Questions



Mathematics, 21.06.2019 20:30


Mathematics, 21.06.2019 20:30


Mathematics, 21.06.2019 20:30

Mathematics, 21.06.2019 20:30




History, 21.06.2019 20:30




Mathematics, 21.06.2019 20:30




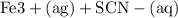 <---->
<---->