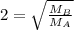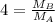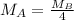At a given temperature and pressure, a sample of Gas A is observed to diffuse twice as fast as a sample of a different gas, B. Based on this: a. The molar mass of A is one fourth that of B b. The molar mass of A is one half that of B c. The molar mass of A is four times that of B d. The molar mass of A is 1.414 times that of B e. The molar mass of A is 0.707 times that of B

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
You know the right answer?
At a given temperature and pressure, a sample of Gas A is observed to diffuse twice as fast as a sam...
Questions

Mathematics, 01.05.2021 01:00

Social Studies, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00


Mathematics, 01.05.2021 01:00


Mathematics, 01.05.2021 01:00


Mathematics, 01.05.2021 01:00





Mathematics, 01.05.2021 01:00

History, 01.05.2021 01:00

History, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00

Computers and Technology, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00

Chemistry, 01.05.2021 01:00

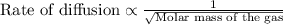
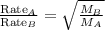
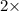 Rate of diffusion of B
Rate of diffusion of B