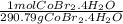Chemistry, 08.04.2020 02:56 sairaanwar67
Calculate the molarity of a solution obtained dissolving 10.0 g of cobalt(Ⅱ) bromide tetrahydrate in enough water to make 450 mL of solution
a. 6.80 × 10-2
b. 7.64 × 10-2
c. 7.64 × 10-5
d. 7.51 × 10-2
e. 0.102 M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Calculate the molarity of a solution obtained dissolving 10.0 g of cobalt(Ⅱ) bromide tetrahydrate in...
Questions

Mathematics, 30.10.2021 21:30


World Languages, 30.10.2021 21:30

Mathematics, 30.10.2021 21:30


Mathematics, 30.10.2021 21:30

Arts, 30.10.2021 21:30

English, 30.10.2021 21:30



Social Studies, 30.10.2021 21:30


Chemistry, 30.10.2021 21:30

English, 30.10.2021 21:30


English, 30.10.2021 21:30