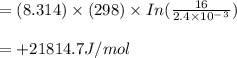Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
A system being studied in a 3.0-liter reactor was charged with 0.150 moles of H2, 0.150 moles of X2,...
Questions

Mathematics, 03.07.2020 23:01



Mathematics, 03.07.2020 23:01

Mathematics, 03.07.2020 23:01



Mathematics, 03.07.2020 23:01



Mathematics, 03.07.2020 23:01




Mathematics, 03.07.2020 23:01





![K_c = \frac{[HX]^2}{[H_2][X_2]}](/tpl/images/0588/7020/cba00.png)
![[H_2] = \frac{0.150}{3} \\= 0.05M](/tpl/images/0588/7020/18e5d.png)
![[X_2] = \frac{0.150}{3} \\= 0.05M](/tpl/images/0588/7020/1b569.png)
![[HX] = \frac{0.600}{3} \\= 0.2M](/tpl/images/0588/7020/2030f.png)
![Q_c = \frac{[HX]^2}{[H_2][X_2]}](/tpl/images/0588/7020/5b1db.png)
![Q_c = \frac{[0.200]^2}{[0.05][0.05]}\\\\=16](/tpl/images/0588/7020/a0039.png)