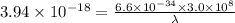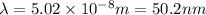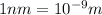Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1

Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
You know the right answer?
The first ionization energy, E , of a helium atom is 3.94 aJ. What is the wavelength of light, in na...
Questions




Mathematics, 17.06.2020 12:57



Mathematics, 17.06.2020 12:57

Mathematics, 17.06.2020 12:57



Mathematics, 17.06.2020 12:57

Mathematics, 17.06.2020 12:57



English, 17.06.2020 12:57

Mathematics, 17.06.2020 12:57

Chemistry, 17.06.2020 12:57



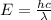
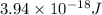
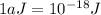
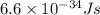
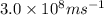
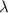 = wavelength of light = ?
= wavelength of light = ?