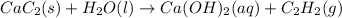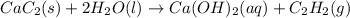Chemistry, 08.04.2020 03:13 natishtaylor1p8dirz
Solid calcium carbide, CaC2, reacts with water to form an aqueous solution of calcium hydroxide and acetylene gas, C2H2. Express your answer as a balanced chemical equation. Identify all of the phases in your answer. nothing

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3
You know the right answer?
Solid calcium carbide, CaC2, reacts with water to form an aqueous solution of calcium hydroxide and...
Questions


Mathematics, 12.03.2020 02:00

Mathematics, 12.03.2020 02:00






Mathematics, 12.03.2020 02:00

History, 12.03.2020 02:00





History, 12.03.2020 02:00