

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1
You know the right answer?
Stock solutions of nitric acid have concentration of 16.0 M. What volume of stock solution is needed...
Questions

History, 21.04.2021 20:30

Mathematics, 21.04.2021 20:30


Mathematics, 21.04.2021 20:30

Physics, 21.04.2021 20:30

Biology, 21.04.2021 20:30


Mathematics, 21.04.2021 20:30

Social Studies, 21.04.2021 20:30

Mathematics, 21.04.2021 20:30



History, 21.04.2021 20:30

Mathematics, 21.04.2021 20:30

Mathematics, 21.04.2021 20:30





Physics, 21.04.2021 20:30

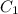 = 16 M
= 16 M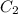 = 2 M
= 2 M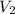 = 2 L = 2000 mL
= 2 L = 2000 mL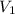
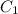 x
x 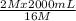 ⇒
⇒ 

