

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
A mixture of three gases has a total pressure of 1,380 mm Hg at 298 K. The mixture is analyzed and i...
Questions




Mathematics, 22.10.2019 18:00


History, 22.10.2019 18:00




History, 22.10.2019 18:00


History, 22.10.2019 18:00

Mathematics, 22.10.2019 18:00

Mathematics, 22.10.2019 18:00

Mathematics, 22.10.2019 18:00

Mathematics, 22.10.2019 18:00

Mathematics, 22.10.2019 18:00

Mathematics, 22.10.2019 18:00

Mathematics, 22.10.2019 18:00

Mathematics, 22.10.2019 18:00

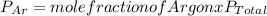
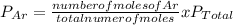
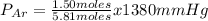
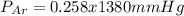
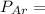 356.04 mmHg
356.04 mmHg

