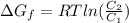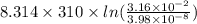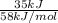Gastric juice (pH 1.5) is produced by pumping HCl from blood plasma (pH 7.4) into the stomach. Calculate the amount of free energy required to concentrate the H in 1 liter of gastric juice at 37 degree of centigrade. Under cellular conditions, how many moles of ATP must be hydrolyzed to provide this amount of free energy

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 23.06.2019 06:40
1.) which of the following is a molecule but not a compound? a.he b.f2 c.h2o d.ch4 2.) what is a physical combination of substances? a.a compound b.a molecule c.a mixture d.an element 3.) what is a chemical combination of substances? a.a compound b.an atom c.a mixture d.an element 4.) what is the relationship between the solute and solvent in a solution? a.they form a compound b.they form a mixture c.they form molecules d.they form chemical bonds 5.) the gases in air dissolve in water. what would be one way to reduce the amount of a gas dissolved in water? a.add more water b.reduce the air pressure c.increase the air pressure d.stir the water 6.) how would you determine the solubility of a substance? a.find how well it dissolved various substances. b.find the mass and the volume of the substance. c.find the temperature at which the substance evaporated. d.find how much i was able to dissolve in a solute. 7.) the periodic table organizes all of the kinds of a.molecules. b.compounds. c.atoms. d.ions. 8.)what distinguishes two substances combined to become a compound vs. two substances combined to become a mixture? a.whether they can be easily separated b.whether they chemically bond together c.whether they both are visible d.whether they are heterogeneous 9.) the principle components of air are: n2 78% o2 21% ar 0.95% co2 0.038% this is a solution of a.molecules and atoms. b.molecules. c.compounds and molecules. d.atoms.
Answers: 1

Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2
You know the right answer?
Gastric juice (pH 1.5) is produced by pumping HCl from blood plasma (pH 7.4) into the stomach. Calcu...
Questions






Mathematics, 19.02.2021 21:20


Mathematics, 19.02.2021 21:20

History, 19.02.2021 21:20


Mathematics, 19.02.2021 21:20

Business, 19.02.2021 21:20



Mathematics, 19.02.2021 21:20



Biology, 19.02.2021 21:20

English, 19.02.2021 21:20

English, 19.02.2021 21:20

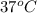 = (37 + 273) K
= (37 + 273) K![-log [H^{+}]](/tpl/images/0588/8520/822be.png)
![[H^{+}]](/tpl/images/0588/8520/85507.png) as follows.
as follows.![[H^{+}] = 10^{-pH}](/tpl/images/0588/8520/241df.png)
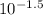
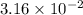 M (
M ( )
)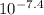
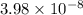 M (
M ( )
)