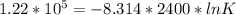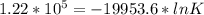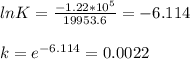Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1


Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
When adjusted for any changes in ΔHΔH and ΔSΔS with temperature, the standard free energy change ΔG∘...
Questions


Mathematics, 14.12.2021 18:30

Geography, 14.12.2021 18:30




English, 14.12.2021 18:30

Mathematics, 14.12.2021 18:30



English, 14.12.2021 18:30

Computers and Technology, 14.12.2021 18:30

Physics, 14.12.2021 18:30



Physics, 14.12.2021 18:30

Social Studies, 14.12.2021 18:30



Mathematics, 14.12.2021 18:30