
The reaction of UO2 with hydrogen ion in aqueous solution 2 UO2 4 H U4 UO22 2 H2O is second order in UO2 and third order overall. Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n... , where '1' is understood for m, n ... (don't enter 1) and concentrations taken to the zero power do not appear. Rate

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2


Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

You know the right answer?
The reaction of UO2 with hydrogen ion in aqueous solution 2 UO2 4 H U4 UO22 2 H2O is second order in...
Questions


Social Studies, 04.09.2020 16:01


Mathematics, 04.09.2020 16:01


Mathematics, 04.09.2020 16:01


Health, 04.09.2020 16:01

Computers and Technology, 04.09.2020 16:01

Spanish, 04.09.2020 16:01

English, 04.09.2020 16:01




Business, 04.09.2020 16:01





![rate=k[UO_2^+]^2[H^+]](/tpl/images/0588/8942/4aa9a.png)
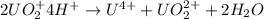
![rate=k[UO_2^+]^n[H^+]^m](/tpl/images/0588/8942/944b7.png)
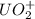 = 2
= 2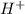 = ?
= ?![rate=k[UO_2^+]^2[H^+]^1](/tpl/images/0588/8942/77310.png)


