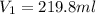Chemistry, 08.04.2020 04:33 damonsmith201615
A chemist must prepare of aqueous calcium sulfate working solution. He'll do this by pouring out some aqueous calcium sulfate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in of the calcium sulfate stock solution that the chemist should pour out. Round your answer to significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1

Chemistry, 23.06.2019 10:30
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2
You know the right answer?
A chemist must prepare of aqueous calcium sulfate working solution. He'll do this by pouring out som...
Questions



Biology, 10.12.2021 19:00


Geography, 10.12.2021 19:00

Mathematics, 10.12.2021 19:00


Mathematics, 10.12.2021 19:00



Mathematics, 10.12.2021 19:00

Mathematics, 10.12.2021 19:00

History, 10.12.2021 19:00

Mathematics, 10.12.2021 19:00

Biology, 10.12.2021 19:00

Mathematics, 10.12.2021 19:00


Mathematics, 10.12.2021 19:00

Business, 10.12.2021 19:00

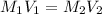
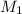 = molarity of stock calcium sulphate solution = 1.82 M
= molarity of stock calcium sulphate solution = 1.82 M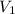 = volume of stock calcium sulphate solution = ?
= volume of stock calcium sulphate solution = ?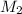 = molarity of dilute calcium sulphate solution = 1.00 M
= molarity of dilute calcium sulphate solution = 1.00 M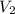 = volume of dilute calcium sulphate solution = 400 ml
= volume of dilute calcium sulphate solution = 400 ml