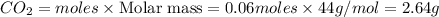G Gaseous methane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 0.96 g of methane is mixed with 6.37 g of oxygen. Calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 11:00
Afraction can be converted to a decimal by dividing the denominator into the numerator. use a calculator. divide to convert the fractions from part a to decimals. then describe the pattern you see in the decimal.
Answers: 3
You know the right answer?
G Gaseous methane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water...
Questions

Mathematics, 06.04.2020 08:56

Mathematics, 06.04.2020 08:56




Mathematics, 06.04.2020 08:57

Mathematics, 06.04.2020 08:57

Mathematics, 06.04.2020 08:57

Mathematics, 06.04.2020 08:57

Mathematics, 06.04.2020 08:58


Chemistry, 06.04.2020 08:58

Mathematics, 06.04.2020 08:59


Mathematics, 06.04.2020 08:59

History, 06.04.2020 08:59

History, 06.04.2020 09:00


Computers and Technology, 06.04.2020 09:00

Chemistry, 06.04.2020 09:00

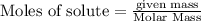
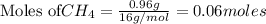
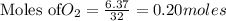
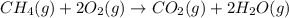
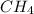 require = 2 moles of
require = 2 moles of 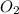
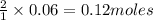 of
of 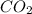
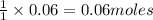 of
of