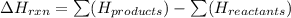Chemistry, 08.04.2020 04:43 pleasehelpme666
Consider the reaction: C2H5OH(ℓ) + 3O2(g) → 2CO2(g) + 3H2O(ℓ); ∆H = –1.37 x 103 kJ Consider the following statements: I. The reaction is endothermic II. The reaction is exothermic. III. The enthalpy term would be different if the water formed was gaseous. Which of these statement(s) is (are) true?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1
You know the right answer?
Consider the reaction: C2H5OH(ℓ) + 3O2(g) → 2CO2(g) + 3H2O(ℓ); ∆H = –1.37 x 103 kJ Consider the foll...
Questions




Mathematics, 16.11.2020 21:20




Mathematics, 16.11.2020 21:20

Mathematics, 16.11.2020 21:20

Health, 16.11.2020 21:20


Mathematics, 16.11.2020 21:20


Biology, 16.11.2020 21:20


Law, 16.11.2020 21:20


Social Studies, 16.11.2020 21:20

Computers and Technology, 16.11.2020 21:20

Biology, 16.11.2020 21:20