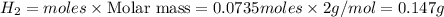Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2

Chemistry, 23.06.2019 05:30
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1
You know the right answer?
Sodium hydroxide reacts with aluminum and water to produce hydrogen gas:2 Al(s) + 2 NaOH(aq) + 6 H2O...
Questions

Mathematics, 12.09.2020 01:01

Chemistry, 12.09.2020 01:01

History, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

English, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

French, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Chemistry, 12.09.2020 01:01

Geography, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

Mathematics, 12.09.2020 01:01

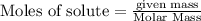
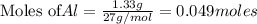
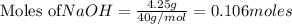
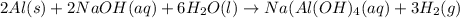
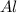 require = 2 moles of
require = 2 moles of ![NaOH/tex]Thus 0.049 moles of [tex]Al](/tpl/images/0589/0248/32375.png) will require=
will require=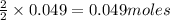 of
of 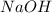
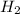
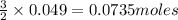 of
of