
Chemistry, 08.04.2020 06:40 cancerbaby209
When 1 mol of methane is burned at constant pressure, −890 kJ/mol of energy is released as heat. If a 3.72 g sample of methane is burned at constant pressure, what will be the value of ∆H? (Hint: Convert the grams of methane to moles. Also make sure your answer has the correct sign for an exothermic process.) Answer in units of kJ.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
You know the right answer?
When 1 mol of methane is burned at constant pressure, −890 kJ/mol of energy is released as heat. If...
Questions


English, 22.04.2021 22:40

Mathematics, 22.04.2021 22:40

Mathematics, 22.04.2021 22:40

Arts, 22.04.2021 22:40

Mathematics, 22.04.2021 22:40

English, 22.04.2021 22:40


Health, 22.04.2021 22:40

English, 22.04.2021 22:40




English, 22.04.2021 22:40


Mathematics, 22.04.2021 22:40

Mathematics, 22.04.2021 22:40

Mathematics, 22.04.2021 22:40


Chemistry, 22.04.2021 22:40

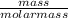
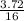 = 0.23moles
= 0.23moles 

