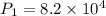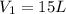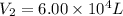Chemistry, 08.04.2020 17:17 clydeben4543
A 15.0 L tank of gas is contained at a high pressure of 8.20*10^4. The tank is opened and the gas expands into an empty chamber with a volume of 6.00*10^4 L. Calculate the new pressure of the gas.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2


Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
A 15.0 L tank of gas is contained at a high pressure of 8.20*10^4. The tank is opened and the gas ex...
Questions


Mathematics, 09.02.2021 19:20

Mathematics, 09.02.2021 19:20

Law, 09.02.2021 19:20




Mathematics, 09.02.2021 19:20


Mathematics, 09.02.2021 19:20

Mathematics, 09.02.2021 19:20



Mathematics, 09.02.2021 19:20

Mathematics, 09.02.2021 19:20


Mathematics, 09.02.2021 19:20

Mathematics, 09.02.2021 19:20

Mathematics, 09.02.2021 19:20

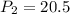
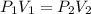 ..........1
..........1