
Chemistry, 08.04.2020 18:39 ligittiger12806
If the number of moles of gas increases to 1.5n, what is the new volume if pressure and temperature are
held constant?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
If the number of moles of gas increases to 1.5n, what is the new volume if pressure and temperature...
Questions


Mathematics, 12.12.2020 17:00

Biology, 12.12.2020 17:00


World Languages, 12.12.2020 17:00

Biology, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Computers and Technology, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

History, 12.12.2020 17:00

History, 12.12.2020 17:00

Biology, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

Advanced Placement (AP), 12.12.2020 17:00

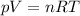
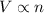
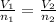
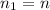 is the initial number of moles
is the initial number of moles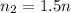 is the final number of moles
is the final number of moles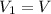 is the initial volume of the gas
is the initial volume of the gas


