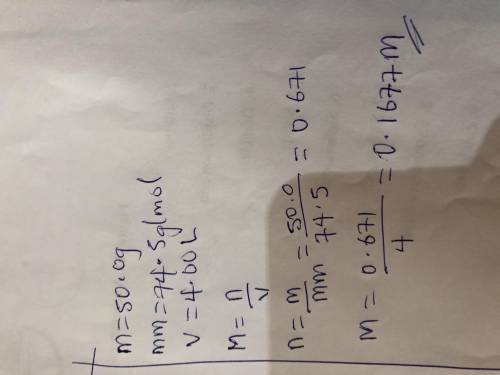50.0 grams of KCl is dissolved in water to make a 4.00 L
solution. What is the molarity of the...

Chemistry, 08.04.2020 19:03 SoccerdudeDylan
50.0 grams of KCl is dissolved in water to make a 4.00 L
solution. What is the molarity of the solution? (Molar mass of
KCl = 74.5 g/mol) ___ M ( Round your answer to the
appropriate number of four significant figures)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which is true of the reactants in this displacement reaction? fe + 2hcl fecl2 + h2 a. the reactants are located to the left of the arrow in the chemical equation. b. the reactants contain 1 iron atom, 2 hydrogen atoms, and 1 chlorine atom. c. the reactants are the atoms, molecules, or compounds formed in the reaction. d. the reactants have the same physical and chemical properties as the products.
Answers: 1


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

You know the right answer?
Questions

Mathematics, 16.12.2021 05:10

Mathematics, 16.12.2021 05:10

Mathematics, 16.12.2021 05:10

Social Studies, 16.12.2021 05:10


Mathematics, 16.12.2021 05:10

Computers and Technology, 16.12.2021 05:10

Social Studies, 16.12.2021 05:10


Mathematics, 16.12.2021 05:10


English, 16.12.2021 05:10

English, 16.12.2021 05:10

Mathematics, 16.12.2021 05:10

Spanish, 16.12.2021 05:10

History, 16.12.2021 05:10



Social Studies, 16.12.2021 05:10