Given the reaction represented by the balanced equation:
CH4(g)+3F2(g)->3HF(g)+CHF3 c...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 08:40
Which statement is true according to the kinetic theory? a. molecules of different gases with the same mass and temperature always have the same average density. b. molecules of different gases with the same mass and temperature always have the same average volume. c. molecules of different gases with the same mass and temperature always have the same pressure. d. molecules of different gases with the same mass and temperature always have the same molecular mass. e. molecules of different gases with the same mass and temperature always have the same average kinetic energy.
Answers: 1

Chemistry, 23.06.2019 10:30
Describe the hybridization of each carbon and nitrogen atom in each of the following structures
Answers: 1

Chemistry, 23.06.2019 13:30
What is one measurement needed to calculate the speed of an object?
Answers: 1
You know the right answer?
Questions


Chemistry, 14.04.2020 19:05

English, 14.04.2020 19:05

Mathematics, 14.04.2020 19:05

Physics, 14.04.2020 19:05






Computers and Technology, 14.04.2020 19:06





Mathematics, 14.04.2020 19:06

Mathematics, 14.04.2020 19:06

Social Studies, 14.04.2020 19:06

History, 14.04.2020 19:06

Computers and Technology, 14.04.2020 19:06

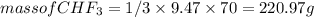
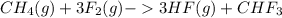
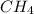 ) is taken excess amount
) is taken excess amount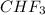 depend only on mass of fluorine
depend only on mass of fluorine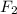 =180 g
=180 g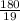 =9.47
=9.47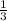 mole of
mole of 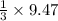 mole of
mole of 

