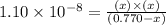Chemistry, 08.04.2020 19:24 jasmine2919
For the reaction below, Kc = 1.10 × 10⁻⁸. What is the equilibrium concentration of OH⁻ if the reaction begins with 0.770 M HONH₂?
HONH₂ (aq) + H₂O (l) ⇌ HONH₃⁺ (aq) + OH⁻ (aq)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
For the reaction below, Kc = 1.10 × 10⁻⁸. What is the equilibrium concentration of OH⁻ if the reacti...
Questions




Computers and Technology, 20.02.2020 22:39





Social Studies, 20.02.2020 22:40

Health, 20.02.2020 22:40


English, 20.02.2020 22:40








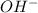 at equilibrium is 0.000092 M
at equilibrium is 0.000092 M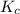
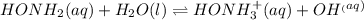
![K_c=\frac{[HONH_3^+]\times [OH^-]}{[HONH_2]}](/tpl/images/0589/8195/4bf94.png)