
Chemistry, 08.04.2020 19:25 angelaisthebest1700
For the reaction H2 S(g) + I2 (s) ⇌ S(s) + 2 HI(g) Kp = 1.33×10–5 at 333 K. What will be the total pressure of the gases above an equilibrium mixture if, at equilibrium, PHI = 0.010 × PH2 S?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 21.06.2019 18:00
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
For the reaction H2 S(g) + I2 (s) ⇌ S(s) + 2 HI(g) Kp = 1.33×10–5 at 333 K. What will be the total p...
Questions



Computers and Technology, 20.09.2020 14:01


Mathematics, 20.09.2020 14:01




Mathematics, 20.09.2020 14:01

Physics, 20.09.2020 14:01


Physics, 20.09.2020 14:01

English, 20.09.2020 14:01







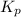
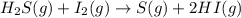
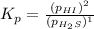
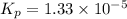
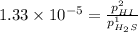
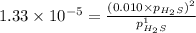
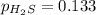
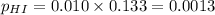
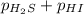 = 0.133+0.0013 = 0.1343 atm
= 0.133+0.0013 = 0.1343 atm

