
Chemistry, 08.04.2020 20:07 Darkknighta26
The reaction 2 n2o5(g) → 4 no2(g) + o2(g) is found to obey the rate law: rate = k [n2o5]. does the reaction mechanism consist of only a single bimolecular step? 1. yes, it must 2. it might, but you cannot tell from the information given. 3. no, it cannot

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
The reaction 2 n2o5(g) → 4 no2(g) + o2(g) is found to obey the rate law: rate = k [n2o5]. does the r...
Questions

Mathematics, 21.12.2019 19:31


Biology, 21.12.2019 19:31




Mathematics, 21.12.2019 20:31

Mathematics, 21.12.2019 20:31

English, 21.12.2019 20:31





Biology, 21.12.2019 20:31






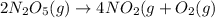
![Rate=k[N_2O_5]^1](/tpl/images/0589/8713/ae447.png)
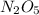 , it is a complex reaction.
, it is a complex reaction.

