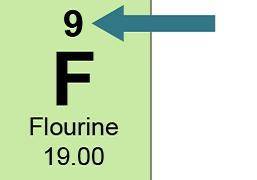
This is how fluorine appears in the periodic table.
A green box has F at the center and...

Chemistry, 08.04.2020 21:09 milkshakegrande101
This is how fluorine appears in the periodic table.
A green box has F at the center and 9 above. Below it says fluorine and below that 19.00. A blue arrow points to 9.
What information does "9” give about an atom of fluorine? Select three options.
the atomic number
the atomic mass
the number of protons
the number of electrons
the number of neutrons

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
Questions






Mathematics, 29.04.2021 17:10

Mathematics, 29.04.2021 17:10

Mathematics, 29.04.2021 17:10

English, 29.04.2021 17:10


Mathematics, 29.04.2021 17:10




Mathematics, 29.04.2021 17:10

Mathematics, 29.04.2021 17:10



Spanish, 29.04.2021 17:10

Chemistry, 29.04.2021 17:10



