
Chemistry, 08.04.2020 21:35 charlesiarenee0
Consider the reaction H2(g) + I2(g) <-> HI(g) with an equilibrium constant of 46.3 and a reaction quotient of 525. Which direction will the system shift to?
The equilibrium will shift to the left to favor the reactants.
The equilibrium will shift to the right to favor the products.
The equilibrium will not shift in any direction.
The equilibrium will shift to the forward reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1


Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Consider the reaction H2(g) + I2(g) <-> HI(g) with an equilibrium constant of 46.3 and a react...
Questions

Mathematics, 02.04.2020 23:59

Mathematics, 02.04.2020 23:59

Mathematics, 02.04.2020 23:59

Spanish, 02.04.2020 23:59






English, 02.04.2020 23:59

Mathematics, 02.04.2020 23:59

History, 02.04.2020 23:59

Mathematics, 02.04.2020 23:59

Mathematics, 02.04.2020 23:59






Mathematics, 02.04.2020 23:59

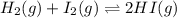
![Q=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0590/2867/236ed.png)
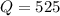

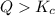 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.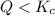 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.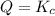 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

