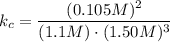Chemistry, 08.04.2020 21:38 mjasmine3280
Upper n subscript 2 (g) plus 3 upper H subscript 2 (g) double-headed arrow 2 upper N upper H subscript 3 (g). At equilibrium, the concentrations of the different species are as follows. [NH3] = 0.105 M [N2] = 1.1 M [H2] = 1.50 M

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
Upper n subscript 2 (g) plus 3 upper H subscript 2 (g) double-headed arrow 2 upper N upper H subscri...
Questions



Physics, 12.08.2020 05:01

Biology, 12.08.2020 05:01

History, 12.08.2020 05:01

Physics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01



Mathematics, 12.08.2020 05:01







Mathematics, 12.08.2020 05:01

Computers and Technology, 12.08.2020 05:01


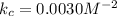
![k_c=\dfrac{[NH_3]^2}{[N_2]\cdot [H_2]^3}](/tpl/images/0590/3046/c4fb6.png)