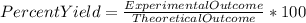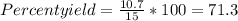Chemistry, 08.04.2020 22:01 GreenHerbz206
When Wolverine’s 10-pound adamantium claws are dissolved in 100 mL of 10 M nitric acid, 10.7 grams of adamantium nitrate are recovered. If we expected 15.0 grams of adamantium nitrate to be recovered in a complete reaction, what was the percent yield?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1
You know the right answer?
When Wolverine’s 10-pound adamantium claws are dissolved in 100 mL of 10 M nitric acid, 10.7 grams o...
Questions

Mathematics, 29.01.2020 22:00

Biology, 29.01.2020 22:00



Mathematics, 29.01.2020 22:00


History, 29.01.2020 22:00

Social Studies, 29.01.2020 22:00

Mathematics, 29.01.2020 22:00





Computers and Technology, 29.01.2020 22:00

English, 29.01.2020 22:00

Business, 29.01.2020 22:00

Mathematics, 29.01.2020 22:00


Geography, 29.01.2020 22:00