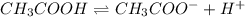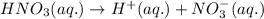Chemistry, 08.04.2020 21:58 rchapman414
Choose the statement below that is TRUE. Question 7 options: The term "strong electrolyte" means that the substance is extremely reactive. The term "weak electrolyte" means that the substance is inert. A weak acid solution consists of mostly nonionized acid molecules. A strong acid solution consists of only partially ionized acid molecules.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2
You know the right answer?
Choose the statement below that is TRUE. Question 7 options: The term "strong electrolyte" means tha...
Questions

Mathematics, 16.04.2020 18:34


Mathematics, 16.04.2020 18:34

Chemistry, 16.04.2020 18:34

Mathematics, 16.04.2020 18:34

Mathematics, 16.04.2020 18:34

Mathematics, 16.04.2020 18:34

History, 16.04.2020 18:34

Chemistry, 16.04.2020 18:34




Biology, 16.04.2020 18:34



Advanced Placement (AP), 16.04.2020 18:34




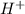 ions when dissolved in water. Thus most of molecules remain unionized in solutions.
ions when dissolved in water. Thus most of molecules remain unionized in solutions.